Researchers
have been able to coax human breast cancer cells to turn into fat cells in a
new proof-of-concept study in mice.
To achieve
this feat, the team exploited a weird pathway that metastasising cancer cells
have; their results are just a first step, but it's a truly promising approach.
When you cut
your finger, or when a foetus grows organs, the epithelium cells begin to look
less like themselves, and more 'fluid' – changing into a type of stem cell
called a mesenchyme and then reforming into whatever cells the body needs.
This process
is called epithelial-mesenchymal transition (EMT) and it's been known for awhile that cancer can use both this one and the opposite pathway called MET
(mesenchymal‐to‐epithelial transition), to spread throughout the body and
metastasise.
The
researchers took mice implanted with an aggressive form of human breast cancer,
and treated them with both a diabetic drug called rosiglitazone and a cancer
treatment called trametinib.
Thanks to
these drugs, when cancer cells used one of the above-mentioned transition
pathways, instead of spreading they changed from cancer into fat cells – a
process called adipogenesis.
"The models used in this study have allowed the evaluation of disseminating cancer cell adipogenesis in the immediate tumour surroundings," the team writes in their paper.
"The
results indicate that in a patient-relevant setting combined therapy with
rosiglitazone and trametinib specifically targets cancer cells with increased
plasticity and induces their adipogenesis."
Although not
every cancer cell changed into a fat cell, the ones that underwent adipogenesis
didn't change back.
"The breast cancer cells that underwent an EMT not only differentiated into fat cells, but also completely stopped proliferating," says senior author GerhardChristofori, a biochemist at the University of Basel, in Switzerland.
"As far
as we can tell from long-term culture experiments, the cancer cells-turned-fat
cells remain fat cells and do not revert back to breast cancer cells."
So how does
this work? Well, as a drug trametinib both increases the transition process of
cells - such as cancer cells turning into stem cells - and then increases the
conversion of those stem cells into fat cells.
Rosiglitazone
was less important, but in combination with trametinib, it also helped the stem
cells convert into fat cells.
"Adipogenic differentiation therapy with a combination of rosiglitazone and [trametinib] efficiently inhibits cancer cell invasion, dissemination, and metastasis formation in various preclinical mouse models of breast cancer," the team writes.
(Department of
Biomedicine, University of Basel)
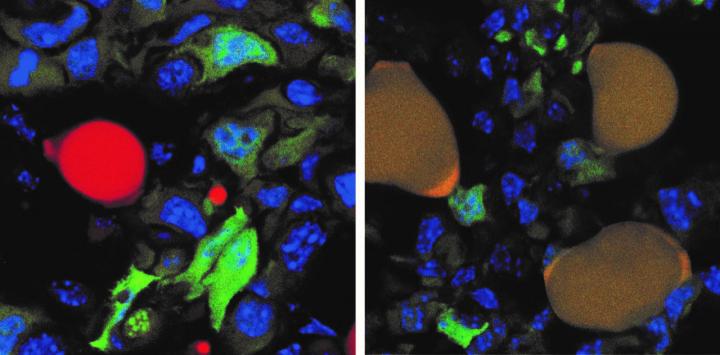
The image
above shows this process, with the cancer cells tagged with a green fluorescent
protein and normal red fat cell on the left. The cancer-turned-fat cells
display as brown (on the right) because the red of the fat cells combines with
the green of the protein cancer cell tag.
What's
exciting is that these two drugs are already FDA-approved, so it should be
easier to get this type of treatment into clinical trials for actual people.
That's
exciting even despite the fact that we know many mouse-tested treatments don't
actually make it to, or fail, the clinical trial stage. The fact this worked on
human cancer cells gives a little extra hope.
In the
meantime, the team is investigating whether this therapy would work combined
with chemotherapy, and whether it would apply to other types of cancers.
"In future, this innovative therapeutic approach could be used in combination with conventional chemotherapy to suppress both primary tumour growth and the formation of deadly metastases," Christofori explained to the PressAssociation.
"The
clinical evaluation of the treatment's repressive effect on experimental breast
cancer metastasis and, thus, of its potential in treating stage IV breast
cancer will require adjuvant combinations with chemotherapy in advanced
preclinical models," the team writes.
"Since
we have used FDA-approved drugs to study the preclinical effect of the
treatment, a clinical translation may be possible."
The research
is published in Cancer Cell.
Comments
Post a Comment